Electrical Conductivity of Various Metals: Difference between revisions
Jump to navigation
Jump to search
Created page with "The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. Thi..." |
(No difference)
|
Revision as of 07:40, 3 December 2018
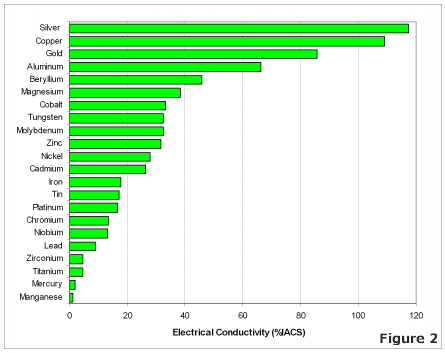
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction.