Electrical Conductivity of Various Metals: Difference between revisions
Jump to navigation
Jump to search
Created page with "The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. Thi..." |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction. | The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction. | ||
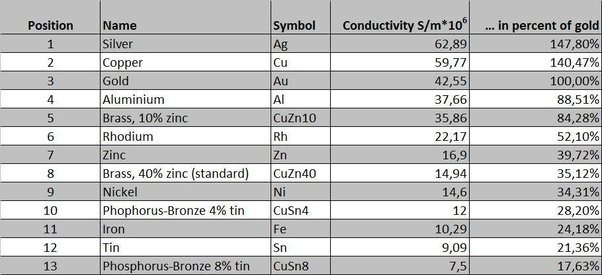
[[File:conductivitymetalchart0.jpg]] | |||
<h3>Conductivity & Resistivity in Metals</h3> | |||
{| border="1"<tbody> | |||
! <h3>Material</h3> | |||
! <h3>Resistivity | |||
p(Ω•m) at 20°C</h3> | |||
! <h3>Conductivity | |||
σ(S/m) at 20°C</h3> | |||
|- | |||
| Silver | |||
| 1.59x10<sup>-8</sup> | |||
| 6.30x10<sup>7</sup> | |||
|- | |||
| Copper | |||
| 1.68x10<sup>-8</sup> | |||
| 5.98x10<sup>7</sup> | |||
|- | |||
| Annealed Copper | |||
| 1.72x10<sup>-8</sup> | |||
| 5.80x10<sup>7</sup> | |||
|- | |||
| Gold | |||
| 2.44x10<sup>-8</sup> | |||
| 4.52x10<sup>7</sup> | |||
|- | |||
| Aluminum | |||
| 2.82x10<sup>-8</sup> | |||
| 3.5x10<sup>7</sup> | |||
|- | |||
| Calcium | |||
| 3.36x10<sup>-8</sup> | |||
| 2.82x10<sup>7</sup> | |||
|- | |||
| Beryllium | |||
| 4.00x10<sup>-8</sup> | |||
| 2.500x10<sup>7</sup> | |||
|- | |||
| Rhodium | |||
| 4.49x10<sup>-8</sup> | |||
| 2.23x10<sup>7</sup> | |||
|- | |||
| Magnesium | |||
| 4.66x10<sup>-8</sup> | |||
| 2.15x10<sup>7</sup> | |||
|- | |||
| Molybdenum | |||
| 5.225x10<sup>-8</sup> | |||
| 1.914x10<sup>7</sup> | |||
|- | |||
| Iridium | |||
| 5.289x10<sup>-8</sup> | |||
| 1.891x10<sup>7</sup> | |||
|- | |||
| Tungsten | |||
| 5.49x10<sup>-8</sup> | |||
| 1.82x10<sup>7</sup> | |||
|- | |||
| Zinc | |||
| 5.945x10<sup>-8</sup> | |||
| 1.682x10<sup>7</sup> | |||
|- | |||
| Cobalt | |||
| 6.25x10<sup>-8</sup> | |||
| 1.60x10<sup>7</sup> | |||
|- | |||
| Cadmium | |||
| 6.84x10<sup>-8</sup> | |||
| 1.46<sup>7</sup> | |||
|- | |||
| Nickel (electrolytic) | |||
| 6.84x10<sup>-8</sup> | |||
| 1.46x10<sup>7</sup> | |||
|- | |||
| Ruthenium | |||
| 7.595x10<sup>-8</sup> | |||
| 1.31x10<sup>7</sup> | |||
|- | |||
| Lithium | |||
| 8.54x10<sup>-8</sup> | |||
| 1.17x10<sup>7</sup> | |||
|- | |||
| Iron | |||
| 9.58x10<sup>-8</sup> | |||
| 1.04x10<sup>7</sup> | |||
|- | |||
| Platinum | |||
| 1.06x10<sup>-7</sup> | |||
| 9.44x10<sup>6</sup> | |||
|- | |||
| Palladium | |||
| 1.08x10<sup>-7</sup> | |||
| 9.28x10<sup>6</sup> | |||
|- | |||
| Tin | |||
| 1.15x10<sup>-7</sup> | |||
| 8.7x10<sup>6</sup> | |||
|- | |||
| Selenium | |||
| 1.197x10<sup>-7</sup> | |||
| 8.35x10<sup>6</sup> | |||
|- | |||
| Tantalum | |||
| 1.24x10<sup>-7</sup> | |||
| 8.06x10<sup>6</sup> | |||
|- | |||
| Niobium | |||
| 1.31x10<sup>-7</sup> | |||
| 7.66x10<sup>6</sup> | |||
|- | |||
| Steel (Cast) | |||
| 1.61x10<sup>-7</sup> | |||
| 6.21x10<sup>6</sup> | |||
|- | |||
| Chromium | |||
| 1.96x10<sup>-7</sup> | |||
| 5.10x10<sup>6</sup> | |||
|- | |||
| Lead | |||
| 2.05x10<sup>-7</sup> | |||
| 4.87x10<sup>6</sup> | |||
|- | |||
| Vanadium | |||
| 2.61x10<sup>-7</sup> | |||
| 3.83x10<sup>6</sup> | |||
|- | |||
| Uranium | |||
| 2.87x10<sup>-7</sup> | |||
| 3.48x10<sup>6</sup> | |||
|- | |||
| Antimony* | |||
| 3.92x10<sup>-7</sup> | |||
| 2.55x10<sup>6</sup> | |||
|- | |||
| Zirconium | |||
| 4.105x10<sup>-7</sup> | |||
| 2.44x10<sup>6</sup> | |||
|- | |||
| Titanium | |||
| 5.56x10<sup>-7</sup> | |||
| 1.798x10<sup>6</sup> | |||
|- | |||
| Mercury | |||
| 9.58x10<sup>-7</sup> | |||
| 1.044x10<sup>6</sup> | |||
|- | |||
| Germanium* | |||
| 4.6x10<sup>-1</sup> | |||
| 2.17 | |||
|- | |||
| Silicon* | |||
| 6.40x10<sup>2</sup> | |||
| 1.56x10<sup>-3</sup></tbody> | |||
|}<div id="billboard3-sticky_1-0" class="comp billboard3-sticky billboard-sticky" data-height="600" data-parent=""><div class="spacer"><div id="billboard3-dynamic_1-0" class="comp billboard3-dynamic mntl-gpt-dynamic-adunit mntl-gpt-adunit gpt billboard dynamic"><div id="billboard3" class="wrapper" data-type="billboard" data-pos="btf2" data-priority="5" data-sizes="[[300, 250], [300, 252], [3, 1], "fluid"]" data-rtb="true" data-targeting="{}"></div></div><!-- end: comp billboard3-dynamic mntl-gpt-dynamic-adunit mntl-gpt-adunit gpt billboard dynamic --></div></div><!-- end: comp billboard3-sticky billboard-sticky --> | |||
*Note: The resistivity of semiconductors (metalloids) is heavily dependent on the presence of impurities in the material. | |||
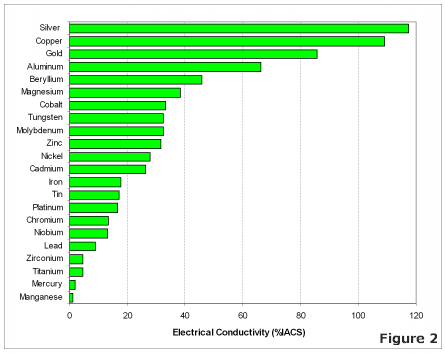
[[File:conductivitymetalchart1.gif]] | [[File:conductivitymetalchart1.gif]] | ||
Latest revision as of 07:52, 3 December 2018
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction.
Conductivity & Resistivity in Metals
Material |
Resistivity p(Ω•m) at 20°C |
Conductivity σ(S/m) at 20°C |
|---|---|---|
| Silver | 1.59x10-8 | 6.30x107 |
| Copper | 1.68x10-8 | 5.98x107 |
| Annealed Copper | 1.72x10-8 | 5.80x107 |
| Gold | 2.44x10-8 | 4.52x107 |
| Aluminum | 2.82x10-8 | 3.5x107 |
| Calcium | 3.36x10-8 | 2.82x107 |
| Beryllium | 4.00x10-8 | 2.500x107 |
| Rhodium | 4.49x10-8 | 2.23x107 |
| Magnesium | 4.66x10-8 | 2.15x107 |
| Molybdenum | 5.225x10-8 | 1.914x107 |
| Iridium | 5.289x10-8 | 1.891x107 |
| Tungsten | 5.49x10-8 | 1.82x107 |
| Zinc | 5.945x10-8 | 1.682x107 |
| Cobalt | 6.25x10-8 | 1.60x107 |
| Cadmium | 6.84x10-8 | 1.467 |
| Nickel (electrolytic) | 6.84x10-8 | 1.46x107 |
| Ruthenium | 7.595x10-8 | 1.31x107 |
| Lithium | 8.54x10-8 | 1.17x107 |
| Iron | 9.58x10-8 | 1.04x107 |
| Platinum | 1.06x10-7 | 9.44x106 |
| Palladium | 1.08x10-7 | 9.28x106 |
| Tin | 1.15x10-7 | 8.7x106 |
| Selenium | 1.197x10-7 | 8.35x106 |
| Tantalum | 1.24x10-7 | 8.06x106 |
| Niobium | 1.31x10-7 | 7.66x106 |
| Steel (Cast) | 1.61x10-7 | 6.21x106 |
| Chromium | 1.96x10-7 | 5.10x106 |
| Lead | 2.05x10-7 | 4.87x106 |
| Vanadium | 2.61x10-7 | 3.83x106 |
| Uranium | 2.87x10-7 | 3.48x106 |
| Antimony* | 3.92x10-7 | 2.55x106 |
| Zirconium | 4.105x10-7 | 2.44x106 |
| Titanium | 5.56x10-7 | 1.798x106 |
| Mercury | 9.58x10-7 | 1.044x106 |
| Germanium* | 4.6x10-1 | 2.17 |
| Silicon* | 6.40x102 | 1.56x10-3</tbody> |
- Note: The resistivity of semiconductors (metalloids) is heavily dependent on the presence of impurities in the material.