Electrical Conductivity of Various Metals: Difference between revisions
Jump to navigation
Jump to search
Created page with "The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. Thi..." |
mNo edit summary |
||
| Line 1: | Line 1: | ||
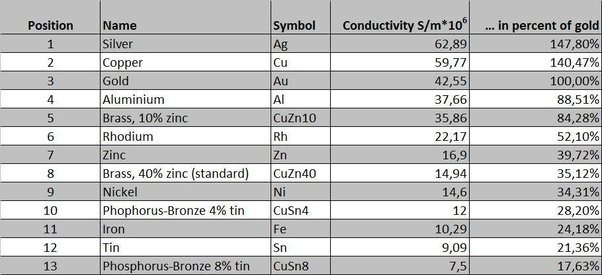
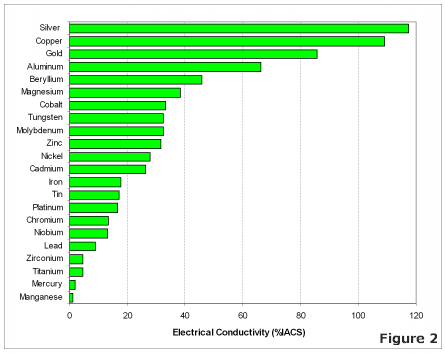
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction. | The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction. | ||
[[File:conductivitymetalchart0.jpg]] | |||
[[File:conductivitymetalchart1.gif]] | [[File:conductivitymetalchart1.gif]] | ||
Revision as of 07:42, 3 December 2018
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction.