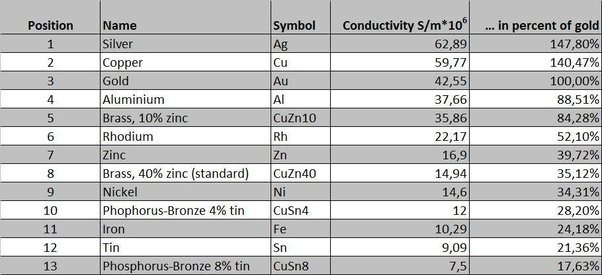
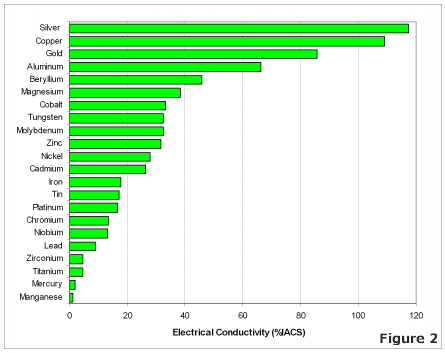
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction.
Conductivity & Resistivity in Metals
Material |
Resistivity p(Ω•m) at 20°C |
Conductivity σ(S/m) at 20°C |
|---|---|---|
| Silver | 1.59x10-8 | 6.30x107 |
| Copper | 1.68x10-8 | 5.98x107 |
| Annealed Copper | 1.72x10-8 | 5.80x107 |
| Gold | 2.44x10-8 | 4.52x107 |
| Aluminum | 2.82x10-8 | 3.5x107 |
| Calcium | 3.36x10-8 | 2.82x107 |
| Beryllium | 4.00x10-8 | 2.500x107 |
| Rhodium | 4.49x10-8 | 2.23x107 |
| Magnesium | 4.66x10-8 | 2.15x107 |
| Molybdenum | 5.225x10-8 | 1.914x107 |
| Iridium | 5.289x10-8 | 1.891x107 |
| Tungsten | 5.49x10-8 | 1.82x107 |
| Zinc | 5.945x10-8 | 1.682x107 |
| Cobalt | 6.25x10-8 | 1.60x107 |
| Cadmium | 6.84x10-8 | 1.467 |
| Nickel (electrolytic) | 6.84x10-8 | 1.46x107 |
| Ruthenium | 7.595x10-8 | 1.31x107 |
| Lithium | 8.54x10-8 | 1.17x107 |
| Iron | 9.58x10-8 | 1.04x107 |
| Platinum | 1.06x10-7 | 9.44x106 |
| Palladium | 1.08x10-7 | 9.28x106 |
| Tin | 1.15x10-7 | 8.7x106 |
| Selenium | 1.197x10-7 | 8.35x106 |
| Tantalum | 1.24x10-7 | 8.06x106 |
| Niobium | 1.31x10-7 | 7.66x106 |
| Steel (Cast) | 1.61x10-7 | 6.21x106 |
| Chromium | 1.96x10-7 | 5.10x106 |
| Lead | 2.05x10-7 | 4.87x106 |
| Vanadium | 2.61x10-7 | 3.83x106 |
| Uranium | 2.87x10-7 | 3.48x106 |
| Antimony* | 3.92x10-7 | 2.55x106 |
| Zirconium | 4.105x10-7 | 2.44x106 |
| Titanium | 5.56x10-7 | 1.798x106 |
| Mercury | 9.58x10-7 | 1.044x106 |
| Germanium* | 4.6x10-1 | 2.17 |
| Silicon* | 6.40x102 | 1.56x10-3</tbody> |
- Note: The resistivity of semiconductors (metalloids) is heavily dependent on the presence of impurities in the material.