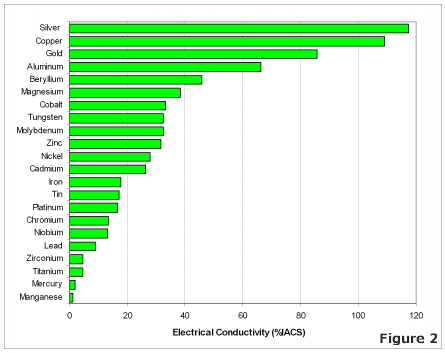
Electrical Conductivity of Various Metals
From Free Knowledge Base- The DUCK Project: information for everyone
The most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong repelling reaction in other electrons. This is the case in the most conductive metals, such as silver, gold, and copper, who each have a single valence electron that moves with little resistance and causes a strong repelling reaction.