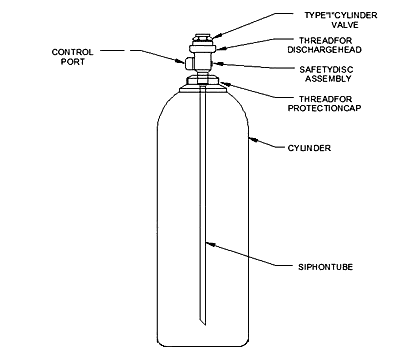
CO2 Cylinder Tank
CO2 tanks come in a range of CO2 cylinder sizes and volumes; from a basic 5lb co2 tank all the way up to a large 50lb co2 tanks. They are designed to contain CO2, a colorless but not odorless gas, consisting of one part carbon and two parts oxygen. At ordinary temperatures, carbon dioxide is relatively unreactive; above 1,700 °C (3,100 °F), it partially decomposes into carbon monoxide and oxygen. Unlike most standard gases, the content of a CO2 cylinder is in a liquid form because, just like propane, it is liquefied under pressure. These tanks should always be stored or used in the upright position.
- CO2 cylinder pressure is approx. 860 psi.
This is most commonly a siphon design, whereas there is a long straw / tube going down the center to allow the liquid to enter and move up and out. This siphon dip tube is what draws the liquid CO2 supply from the cylinder.
construction
Typical CO2 tank cylinders used for various purposes such as carbonation in beverages, firefighting, or industrial applications are often constructed out of aluminum. Aluminum is favored for CO2 cylinders due to its lightweight nature, corrosion resistance, and ability to withstand high pressures. Additionally, aluminum cylinders are generally more affordable and easier to manufacture compared to other materials such as steel or composite materials.
Some CO2 cylinders are constructed using composite materials, such as fiberglass-wrapped aluminum or carbon fiber. These cylinders offer a combination of lightweight construction and high strength. Composite cylinders are often used in applications where weight reduction is critical, such as portable or aerospace applications.
Steel and stainless steel CO2 cylinders too exist. Steel cylinders are commonly used for storing compressed gases, including CO2. While less common than carbon steel cylinders, stainless steel cylinders are sometimes used for storing CO2, especially in industrial or corrosive environments. Stainless steel cylinders are highly durable and resistant to corrosion, making them suitable for long term use in harsh conditions.
alternative uses
Information presented under alternative uses includes factual data and some conjecture including assessments about possible risks. It is neither suggested nor recommended that a CO2 tank cylinder be used for any purpose other than the intended design. An exploration of "what would" or "what could" happen is only theoretical for the purposes of thought and discussion. This web resources nor any of the contributors shall be held liable for what a reader may attempt to do. Do not try this at home.
People will ask if a CO2 cylinder can be repurposed. Some have questioned if such a cylinder can be used as a tank to store compressed air. Others have asked about storing other gasses in such a tank, even propane, which is a highly flammable gas that is stored in pressurized liquid form.
As compared to other types of tanks, specifically, for example, a propane tank: CO2 cylinders have much thicker walls than propane cylinders. Propane cylinders are 250 p.s.i. max pressure, CO2 cylinders are normally 1800 p.s.i. At 100 degrees F. propane exerts about 170 p.s.i., while CO2 exerts about 1400 p.s.i. Even at room temperature a full CO2 cylinders runs about 850 p.s.i. A typical air compressor won't reach much above 120-150 psi.
Unlike propane storage tanks, the CO2 tank lacks safety mechanisms for dealing with flammable gas and excess pressure buildup. CO2 cylinders are typically designed and manufactured to withstand the pressure and properties of carbon dioxide and composed of material that is non-reactive with CO2. Again, as an example, Propane could potentially react with the materials used in the construction of the CO2 cylinder, leading to corrosion, degradation, or even rupture of the cylinder. Further research is required to learn if the material used to construct a CO2 tank can safely contain propane without reaction and/or corrosion.
Some people have devised ways of using an air compressor to refill a CO2 tank. A typical air compressor won't reach much above 120-150 psi which they believe is well within tolerances. This strategy might be so that an individual can store and use compressed air away from a stationary installed compressor, such as filling a flat tire on a large automobile somewhere remote. Compressed air remains air, a gas, and the CO2 tank is designed to store liquid, not gas. This practice could be dangerous and could lead to a tank rupture and injury to the operator.